Try out PMC Labs and tell us what you think. Learn More.

COVAX-19Ⓡ Vaccine: Completely blocks virus transmission to non-immune individuals
Vivek P. Chavda
aDepartment of Pharmaceutics and Pharmaceutical Technology, L M College of Pharmacy, Ahmedabad - 380009, Gujarat, India
Lalitkumar K. Vora
bSchool of Pharmacy, Queen's University Belfast, 97 Lisburn Road, BT9 7BL, UK
Disha R. Vihol
cPharmacy Section, L.M. College of Pharmacy, Ahmedabad - 380058, Gujarat, India
Associated Data
Abstract
Various vaccine platforms are geared against COVID-19 vaccine development to produce immunogens in cells. To design a recombinant protein-based COVID-19 vaccine, Vaxine pty Ltd used computer models of the spike protein and its human receptor, ACE2, to identify how the virus infects human cells. Based on this, the COVAX-19Ⓡ vaccine is synthesized. It does reduce not only COVID-19 disease but also blocks virus shedding and transmission. Researchers are optimistic that this vaccine candidate could be clinically available soon with sufficient vaccine efficacy with a considerable amount of reduction in vaccination-related side effects.
1. Background
Currently, the world is dealing with the SARS-CoV-2 pandemic, and the vaccine is the prominent option to fight against it. Around 119 vaccine candidates are under development, and 15 vaccines are approved for COVID-19. There is increasing demand for newer safe and efficacious candidates as the currently practiced vaccines are proved to be ineffective against the new variants of the viral strain dominated in the different parts of the world. There are a total of 20 vaccine candidates approved for emergency use while 131 vaccine candidates are under development with 383 ongoing clinical trials.
2. COVAX-19Ⓡ Vaccine
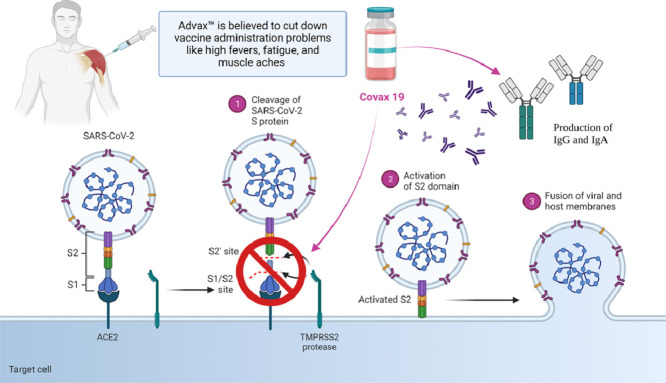
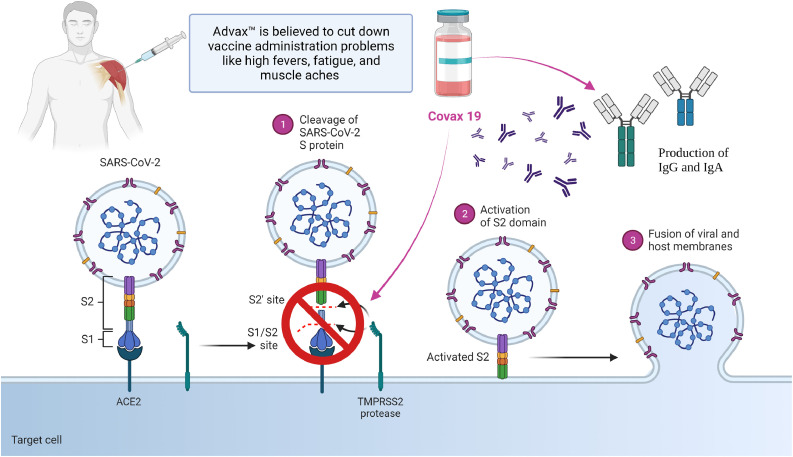
Because of EUA (Emergency Use Authorization), long term safety and efficacy data of vaccines are not available, and there are potentials risk of outbreak (Table 1 ). Apart from that, the vaccines available currently are not on par with the treatment against rapidly mutating covid virus. Additionally, the immunization provided by them is not more than a year. The vaccines cannot stop the disease transmission, leading to widespread health, social, and economic disruption (Li et al., 2020). To overcome these problems, Professor Nikolai Petrovsky of Flinders University and Research Director at Vaxine Pty Ltd of Adelaide, South Australia, developed a novel synthetic vaccine called COVAX-19Ⓡ. It consists of a harmless insect cell-based recombinant spike protein of SARS-CoV–2 in combination with Vaxine's proprietary non-inflammatory Advax™, a polysaccharide adjuvant derived from delta inulin. The immunity against SARS-CoV-2 is because of the generation of neutralizing antibodies and T cells that prevent the virus from attaching to the human angiotensin-converting enzyme 2 (ACE2) receptor in the respiratory epithelium (Fig. 1 ) (Vaxine Pty Ltd 2020).
Table 1
Commercialized vaccine candidates against COVID-19 and their pitfalls.
| Vaccine Platform | Name of Vaccine Candidate | Innovator | Target Moiety | Pitfalls | References |
| mRNA Based Vaccine | mRNA-1273 | Moderna, USA | Nucleoside-modified mRNA (modRNA) encoding a spike protein of SARS-CoV-2 | Limited efficacy against beta and delta variant and requires booster dose. Anaphylactic reactions were also reported in 2.5 % of the cases. Inflammation of The Covering of The Heart or Pericardium. | (Shimabukuro et al., 2021) |
| BNT162b2 (Tozinameran, Comirnaty) | Pfizer/BioNTech, USA and Germany | Nucleoside-modified mRNA (modRNA) encoding a mutated form of the full-length spike protein of SARS-CoV-2 | It requires ultralow temperature storage. Limited efficacy against beta and delta variant and booster dose needed. Vaccination-related side effects were reported. | (Polack et al., 2020) | |
| TAK-919 | Takeda, Japan | mRNA-1273.351, that could be used as a booster shot against the Beta variant | Limited efficacy against delta variant and requires booster dose. Vaccination-related side effects were reported. | (Moderna's vaccine update 2021) | |
| Protein Subunit Vaccine | ZF2001 (RBD-Dimer) | Anhui Zhifei Longcom, China | An adjuvanted protein subunit containing CoV spike receptor-binding domain (RBD) | Limited efficacy and requires booster dose even after 2 doses. | (Yang et al., 2021) |
| EpiVacCorona | The foundation for Biomedical Research and Innovation at Kobe (FBRI), Japan | Having three synthetic viral peptides (One Spike, One N protein and one bacterial peptide) that are conjugated to a large carrier protein | Limited efficacy and requires booster dose even after 2 doses. | (Dobrovidova, 2021) | |
| Adeno viral vector based vaccine | Covishield | Serum Institute of India | ChAdOx1 vector encode for Viral Spike Protein | Many reports of serious allergic reactions to this vaccines were documented. | (Mohammad S Razai and Osama, 2021) |
| Vaxzevria (AZD1222) | Oxford/AstraZeneca, UK | ChAdOx1 vector encode for Viral Spike Protein | 234 reports of serious allergic reactions to AstraZeneca vaccines. 30 cases of anaphylaxis have been reported for the Oxford–AstraZeneca vaccine | (Mohammad S Razai and Osama, 2021, Shimabukuro et al., 2021) | |
| Ad26COVS1, JNJ-78436735 | Janssen (Johnson & Johnson), USA | Ad26 vector encode for Viral Spike Protein | Rare blood-clotting disorder after the delivery and comparatively less efficacious | (Viswanath, 2021) | |
| Gam-COVID-Vac | Gamaleya: Sputnik V, Russia | Ad26 vector encode for Viral Spike Protein and booster dose with Ad5 vector encode for Viral Spike Protein | Requires frozen storage, comparatively less efficacious, and multiple dosing are needed. | (Logunov et al., 2021) | |
| Ad5-nCoV | CanSino, China | Ad5 vector encode for Viral Spike Protein | |||
| Inactivated Vaccine | CoronaVac | Sinovac Pharma Inc., China | Inactivated whole virus i.e. SARS-CoV-2 | Requires frozen storage, comparatively less efficacious and multiple dosing is required. | (Han et al., 2021) |
| BBIBP-CorV | Sinopharma, Beijing, China | Requires booster dose and vaccination-related side effects reported. | (Al Kaabi et al., 2021) | ||
| WIBP-CorV | Sinopharma, Wuhan, China | Requires booster dose and vaccination-related side effects reported. | (Al Kaabi et al., 2021) | ||
| SARS-CoV-2 Vaccine (Vero Cells) | Minhai Biotechnology Co., Beijing, China | Requires booster dose and vaccination-related side effects reported. | (Zhang et al., 2021) | ||
| QazVac | Research Institute for Biological Safety Problems in Kazakhstan | Requires booster dose and vaccination-related side effects reported. | (Batyrov, 2021) | ||
| KoviVac | Chumakov Center, Russia | Requires booster dose and vaccination-related side effects reported. Less effective against the variants of SARS-CoV-2. | (Mir, 2021) | ||
| Covaxin | Bharat Biotech, India | Neurological problems reported, requires booster dose and vaccination-related side effects reported. Less effective against the variants of SARS-CoV-2. | (Thiagarajan, 2021) | ||
Further, the use of an anti-inflammatory adjuvant, Advax™ (GMP-grade delta-inulin), is believed to cut down vaccine administration problems like high fevers, fatigue, and muscle aches which are commonly observed in the currently approved vaccine (Petrovsky, 2020). The team identified and confirmed the role of SARS-CoV-2’s spike protein with the help of Etaluma Luma Scope LS620 provided by AXT Pty Ltd (Chai, 2021). For determining the targets, the team utilized oracle cloud-based supercomputing and artificial intelligence (AI) to develop COVAX-19Ⓡ in only five weeks, which usually takes around 15 years.
Along with their target for COVAX-19Ⓡ, they also found out around 80 possible targets against SARS-CoV-2 and made it available to the world for research (Piplani et al., 2020). For carrying out product and process development, clinical trial programs, and commercial scale-up for the Australian and Asian markets, the company signed a memorandum of understanding with highly experienced GMP manufacturing firm Medytox biopharma of South Korea (Vaxine and Medytox partner on Covid-19 vaccine development 2020). Furthermore, APC, an Irish pharmaceutical research firm that designs, develops, and distributes patented engineering platform technologies to minimize time, cost, and risk in developing medications, collaborated with Vaxine to expedite the development and marketing of COVAX-19Ⓡ (Taylor, 2021).
COVAX-19Ⓡ moved to Phase I clinical trial after obtaining long-lasting protection with superior safety and tolerability results from pre-clinical studies on mice, ferret, and monkey that are conducted in collaboration with the University of Georgia, U.S (Petrovsky, 2020). The Phase I trial was also undertaken independently by the PARC clinical trial team, a University of Adelaide-based research group at the Royal Adelaide Hospital, including 40 healthy participants aged between 18 and 65 years ({"type":"clinical-trial","attrs":{"text":"NCT04453852","term_id":"NCT04453852"}}NCT04453852). The participants were randomized on a 3:1 ratio to receive two intramuscular doses three weeks apart of either active vaccine at a dose of 25 µg spike protein plus 15 mg Advax™ and 0.15mg CpG55.2 (30 participants) or saline placebo (10 participants) (Vaxine Pty Ltd 2020). Phase II trial is going on in Iran at Espinas Palace Hotel, Tehran, with the enrolment of 400 participants (18 and 65 years) to evaluate the immunogenicity and safety (IRCT20150303021315N23), which is a two-armed double-blinded placebo-controlled study. The results are not yet declared in the detailed form, but the team has ensured that the vaccine is very safe and tolerable, along with no transmission of Covid-19 between the individuals. They are also suggesting that COVAX-19Ⓡ will provide effective herd immunity. According to the company's recent news, COVAX-19Ⓡ vaccine will enter into phases II and III clinical trials with a cooperation agreement with Iran. And if these studies are successful, the vaccine will roll out in Iran under the brand name “SpikoGenⓇ” by the CinnaGen Company (SpikogenⓇ, 2021). As compared to the other emergency-approved vaccine candidates against SARS-COV-2 infection, the COVAX-19Ⓡ leads to lesser vaccine administration-related side effects due to the anti-inflammatory adjuvant present in the vaccine formulation. The anti-inflammatory adjuvant and synthetic viral spike protein elements in the vaccine formulation also indicate this vaccine candidate's better safety margin and tolerability. Further, Nikolai Petrovsky expects the vaccine to be effective on new mutant strains of South Africa (Beta Variant), Brazil (Gamma Variant), and India (Delta Variant), with immunity lasting for at least two years.
CRediT authorship contribution statement
Vivek P Chavda has prepared the backbone of the manuscript, wrote the original draft of the manuscript with Disha Vihol. Lalitkumar K Vora and Vivek P Chavda refined the draft, and all authors approved the submitted version. The figure is created with BioRender.com
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., Al Nusair M., Hassany M., Jawad J.S., Abdalla J., Hussein S.E., Al Mazrouei S.K., Al Karam M., Li X., Yang X., Wang W., Lai B., Chen W., Huang S., Wang Q., Yang T., Liu Y., Ma R., Hussain Z.M., Khan T., Saifuddin Fasihuddin M., You W., Xie Z., Zhao Y., Jiang Z., Zhao G., Zhang Y., Mahmoud S., ElTantawy I., Xiao P., Koshy A., Zaher W.A., Wang H., Duan K., Pan A., Yang X. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021 doi: 10.1001/jama.2021.8565. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Batyrov Azamat. 2021. Kazakhstan Launches Production of First Homegrown Vaccine. ‘QazVac,’ (n.d.) April 25. https://caspiannews.com/news-detail/kazakhstan-launches-production-of-first-homegrown-vaccine-qazvac-2021-4-25-0/ (accessed June 18, 2021) [Google Scholar]
- Chai Cameron S. AXT Assists Vaxine in the Search for a COVID-19 Vaccine. Etradewire. 2021 https://etradewire.com/news/axt-assists-vaxine-in-the-search-for-covid-19-vaccine (accessed May 22, 2021) [Google Scholar]
- Dobrovidova O. Latest Russian vaccine comes with a big dose of mystery. Science. 2021;372:116–117. doi: 10.1126/science.372.6538.116. [PubMed] [CrossRef] [Google Scholar]
- B. Han, Y. Song, C. Li, W. Yang, Q. Ma, Z. Jiang, M. Li, X. Lian, W. Jiao, L. Wang, Q. Shu, Z. Wu, Y. Zhao, Q. Li, Q. Gao, Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial, Lancet Infect. Dis. (2021). doi:10.1016/S1473-3099(21)00319-4. [PMC free article] [PubMed] [CrossRef]
- Li Y., Tenchov R., Smoot J., Liu C., Watkins S., Zhou Q. A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development. ACS Cent. Sci. 2021;7:512–533. doi: 10.1021/acscentsci.1c00120. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V, Shcheblyakov D.V, Tukhvatulin A.I., Zubkova O.V, Dzharullaeva A.S., Kovyrshina A.V, Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V, Shcherbinin D.N., Semikhin A.S., Simakova Y.V, Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V, Naroditsky B.S., Gintsburg A.L. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet (London, England) 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Mir Russkiy. 2021. Developer: Kovivac Is Effective Against All Covid-19 Mutations. https://russkiymir.ru/en/news/287084/ (accessed June 16, 2021) [Google Scholar]
- Moderna’s vaccine update, (2021) January 25th, 2021. https://investors.modernatx.com/static-files/1f770088-5909-457b-af99-7ff2454ba28a (accessed June 18, 2021).
- Mohammad S Razai A.M., Osama Tasnime. Covid-19 vaccine adverse events: balancing monitoring with confidence in vaccines. BMJ Opin. 2021 [Google Scholar]
- Petrovsky Nikolai. 2020. Vaxine and University of Georgia Completes First Stage of COVID-19 Ferret Challenge Studies, Einpresswire. https://vaxine.net/vaxine-and-university-of-georgia-complete-first-stage-of-covid-19-ferret-challenge-studies/ (accessed May 22, 2021) [Google Scholar]
- Piplani S., Singh P., Petrovsky N., Winkler D.A. 2020. Computational screening of repurposed drugs and natural products against SARS-Cov-2 main protease (Mpro) as potential COVID-19 therapies. http://arxiv.org/abs/2009.00744 (accessed May 22, 2021) [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V, Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shimabukuro, et al. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine - United States. December 21, 2020-January 10. MMWR. Morb. Mortal. Wkly. Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Shimabukuro T.T., Cole M., Su J.R. Reports of Anaphylaxis after Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA - J. Am. Med. Assoc. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [PubMed] [CrossRef] [Google Scholar]
- SpikogenⓇ A Joint. 2021. Venture Between Vaxine And Cinnagen.https://vaxine.net/spikogen-a-joint-venture-between-vaxine-and-cinnagen/ (accessed May 22, 2021) [Google Scholar]
- Taylor Charlie. 2021. Pharma research firm APC partners to develop Covid-19 vaccine. https://www.irishtimes.com/business/health-pharma/pharma-research-firm-apc-partners-to-develop-covid-19-vaccine-1.4275430 (accessed May 22, 2021) [Google Scholar]
- Thiagarajan K. What do we know about India's Covaxin vaccine? BMJ. 2021;373:n997. doi: 10.1136/bmj.n997. [PubMed] [CrossRef] [Google Scholar]
- Vaxine and Medytox partner on Covid-19 vaccine development Pharm. Technol. 2020 https://www.pharmaceutical-technology.com/news/vaxine-medytox-covid-19-vaccine/ (accessed May 22, 2021) [Google Scholar]
- Vaxine Pty Ltd . ClinicalTrials.gov Identifier: NCT04453852; 2020. Phase 1 Cilinical trials on Monovalent Recombinant COVID19 Vaccine (COVAX19) https://clinicaltrials.gov/ct2/show/{"type":"clinical-trial","attrs":{"text":"NCT04453852","term_id":"NCT04453852"}}NCT04453852 (accessed May 22, 2021) [Google Scholar]
- Viswanath PILLA. Money Control; 2021. Rare clot incidents linked to J&J, AstraZeneca COVID-19 vaccines bring adenovirus vector platform under scanner.https://www.moneycontrol.com/news/business/companies/rare-clot-incidents-linked-to-jj-astrazeneca-covid-19-vaccines-bring-adenovirus-vector-platform-under-scanner-6774801.html (accessed May 14, 2021) [Google Scholar]
- Yang S., Li Y., Dai L., Wang J., He P., Li C., Fang X., Wang C., Zhao X., Huang E., Wu C., Zhong Z., Wang F., Duan X., Tian S., Wu L., Liu Y., Luo Y., Chen Z., Li F., Li J., Yu X., Ren H., Liu L., Meng S., Yan J., Hu Z., Gao L., Gao G.F. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00127-4. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Y. Zhang, G. Zeng, H. Pan, C. Li, Y. Hu, K. Chu, W. Han, Z. Chen, R. Tang, W. Yin, X. Chen, Y. Hu, X. Liu, C. Jiang, J. Li, M. Yang, Y. Song, X. Wang, Q. Gao, F. Zhu, Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial, Lancet Infect. Dis. 21 (2021) 181–192. doi:10.1016/S1473-3099(20)30843-4. [PMC free article] [PubMed] [CrossRef]
Formats:
- Article |
- PubReader |
- ePub (beta) |
- PDF (722K) |
- Cite
-
COVAX-19Ⓡ Vaccine: Completely blocks virus transmission to non-immune individual...COVAX-19Ⓡ Vaccine: Completely blocks virus transmission to non-immune individualsElsevier Public Health Emergency Collection. 2021 Dec; 1(1)100004
Your browsing activity is empty.
Activity recording is turned off.
See more...
 Facebook
Facebook
 Twitter
Twitter
 Google+
Google+